

The term metalloid originally referred to nonmetals. The electrical properties of silicon and germanium enabled the establishment of the semiconductor industry in the 1950s and the development of solid-state electronics from the early 1960s. They and their compounds are used in alloys, biological agents, catalysts, flame retardants, glasses, optical storage and optoelectronics, pyrotechnics, semiconductors, and electronics. Metalloids are usually too brittle to have any structural uses. Most of their other physical properties and chemical properties are intermediate in nature. Chemically, they behave mostly as nonmetals. Typical metalloids have a metallic appearance, but they are brittle and only fair conductors of electricity. Some periodic tables include a dividing line between metals and nonmetals, and the metalloids may be found close to this line. On a standard periodic table, all eleven elements are in a diagonal region of the p-block extending from boron at the upper left to astatine at lower right. Five elements are less frequently so classified: carbon, aluminium, selenium, polonium and astatine. The six commonly recognised metalloids are boron, silicon, germanium, arsenic, antimony and tellurium. Despite the lack of specificity, the term remains in use in the literature of chemistry. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids.
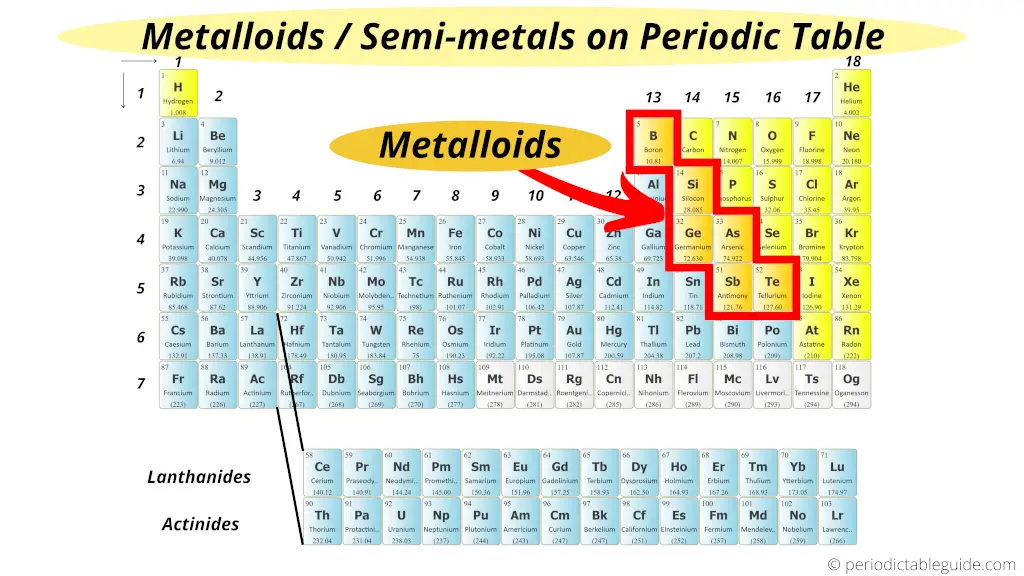
A metalloid is a type of chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals.


 0 kommentar(er)
0 kommentar(er)
